- Products
- Brighter future, by specialized research.

- Functional ingredients for healthy functional food approved by the Ministry of Food and Drug Safety, No. 2023-31
- Saururus chinensis extract (LHF618®)
- Saururus chinensis
-
- Ingredient name
- Saururus chinensis extracts (LHF618®)
-

- Origin
- Saururus chinensis
-

- Daily dose
- 450mg/day
-

- Appearance
- A yellowish-brown powder with
a unique flavor and aroma
-
Features
‘Saururus chinensis extract(LHF618)’, No. 2023-31, is approved as a functional ingredient for healthy functional foods by the Ministry of Food and Drug Safety.
It may help to improve nasal conditions caused by hypersensitivity reactions.
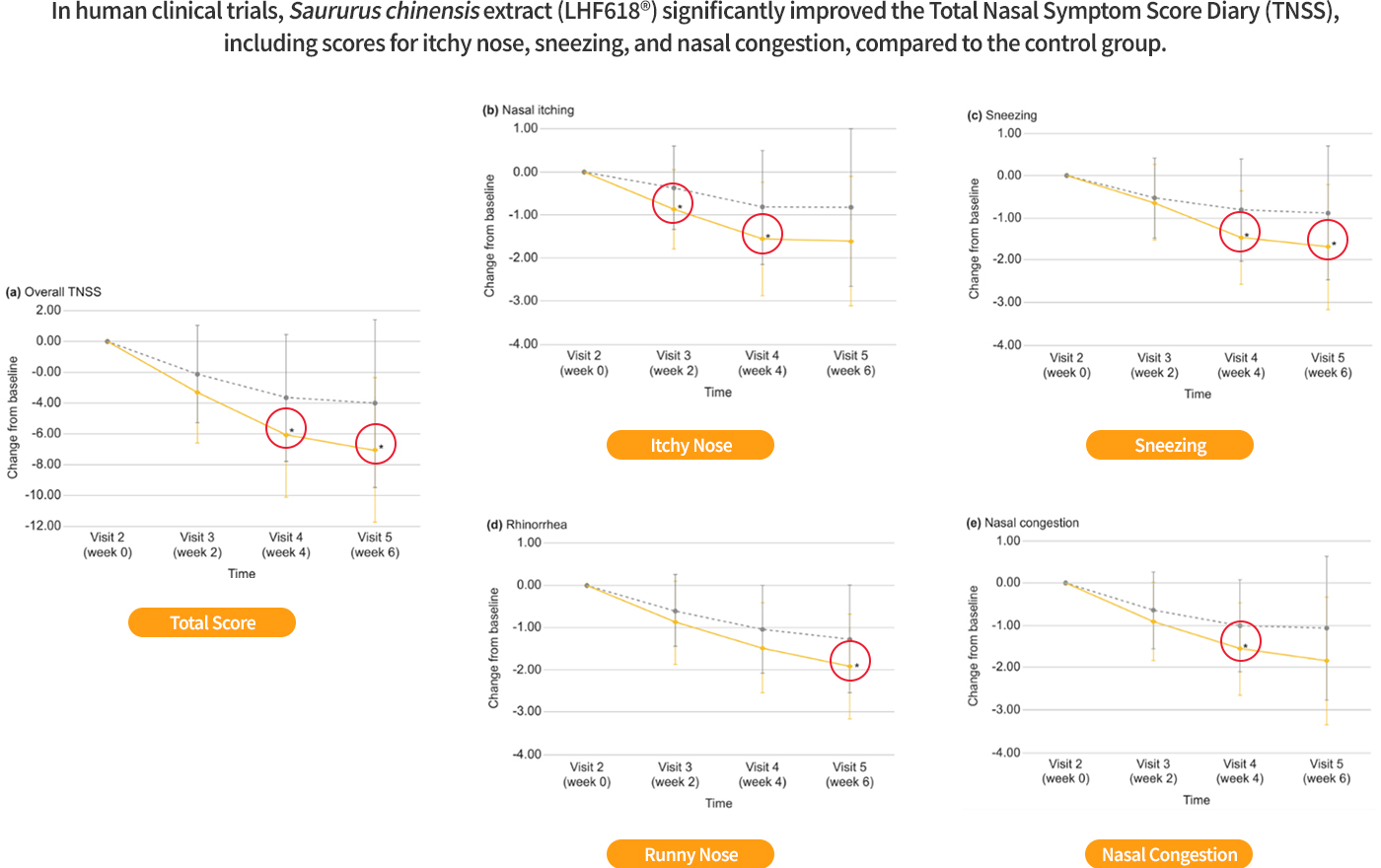
In human clinical trials, compared to the control group, the Total Nasal Symptom Score Diary (TNSS) overall score, nasal itching, sneezing, and nasal congestion showed improvement.
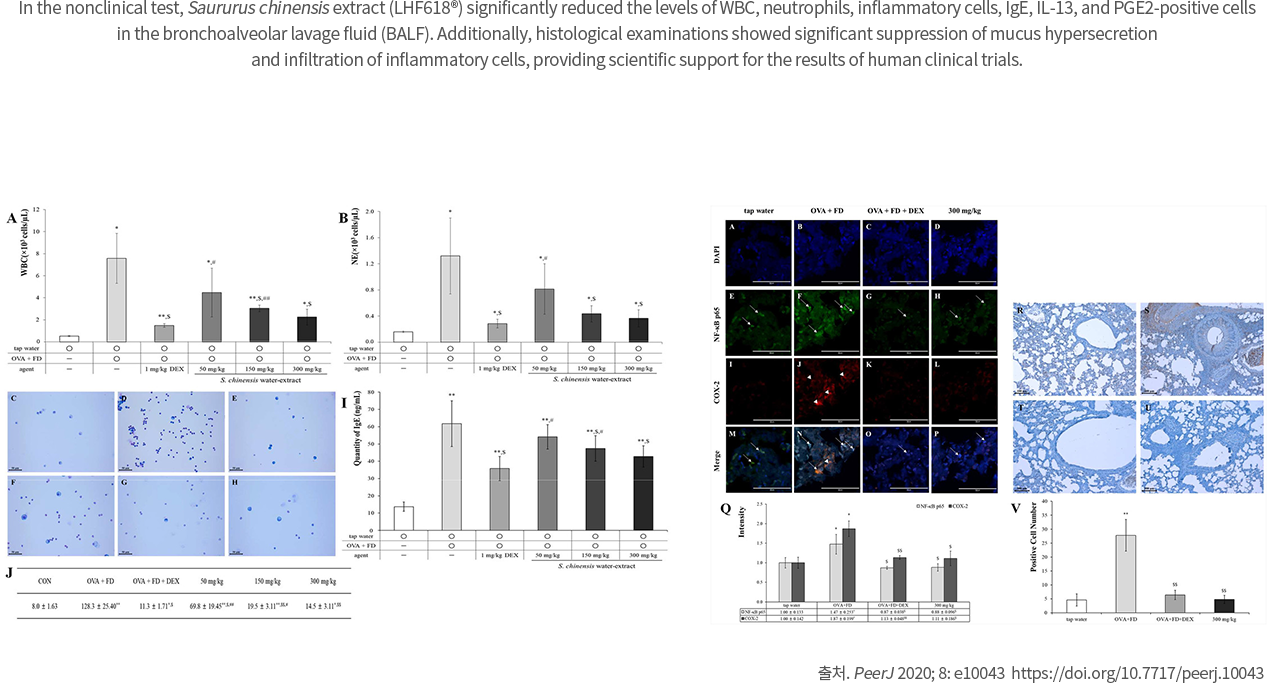
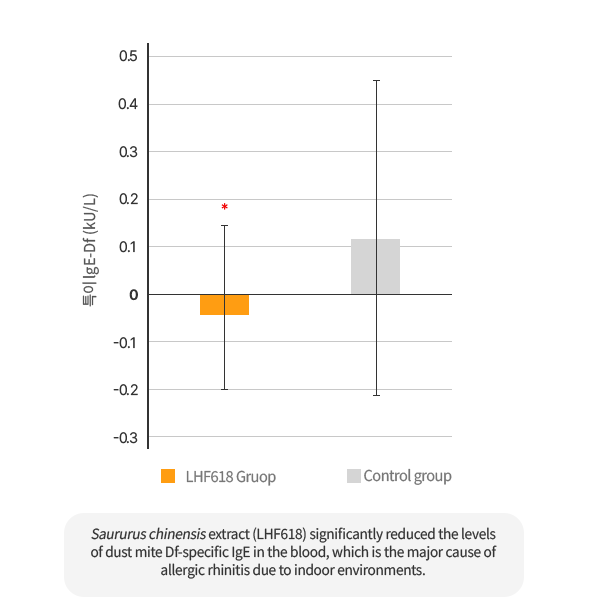
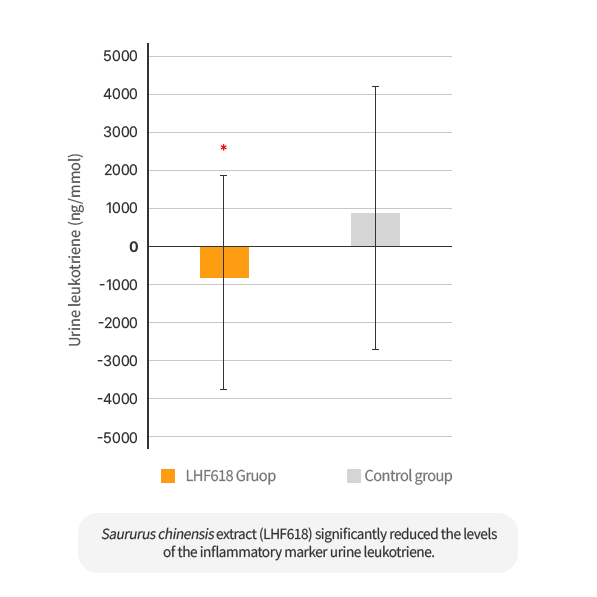
The intake of Saururus Chinensis Extract (LHF618) significantly reduced Dermatophagoides farina specific IgE in the blood and significantly decreased the inflammatory marker, urine leukotriene.
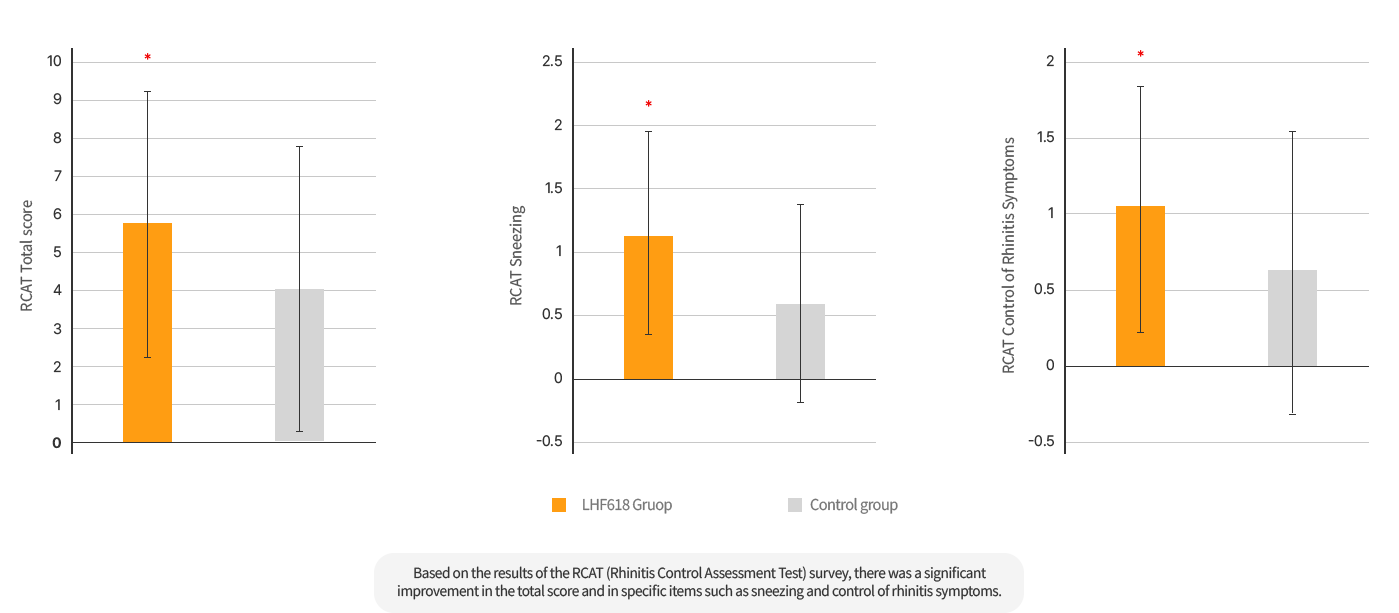
According to the Rhinitis Control Assessment Test (RCAT) survey results, the total score and specific items such as sneezing, and control of rhinitis symptoms were significantly improved.
In allergy skin reaction tests, Tree pollen mixture 1 showed a significant reduction.
-
2023 Approved as a functional ingredient for healthy functional foods by the Ministry of Food and Drug Safety, No. 2023-31
2022 Clinical Trial Completed for Improving Allergic Rhinitis Symptoms
2020 Launch Respiratory Health Drink ‘Phyto Block®’
2019 1st for the 2019 Patent Technology Award by the Korean Intellectual Patent Office (LHF618®)
2019 Bioactive material commercialization project (commercializing the product prevent particular matter)
2019 Korea SMEs and starups agency commercialization project (functional food and drug on respiratory diseases)
2018 ministry of SMEs and startups R&D project
2018 Co-research with Dongshin University
2018 Licensing-in from Yeungnam University
-
Patent
10-2366763: Food composition for the improvement of respiratory health.
10-0675618: Composition for the prevention or treatment of asthma or allergic diseases, including Saururus chinensis extract.
-
Paper
2024 J. Functional Foods 112, 115930
2020 Peer J Sep 24;8:e10043
2010 Journal of Ethnophamacology 132, 143-149
2006 Biological & Pharmaceutical Bulletin 29, 211-215